Properties
Products Properties
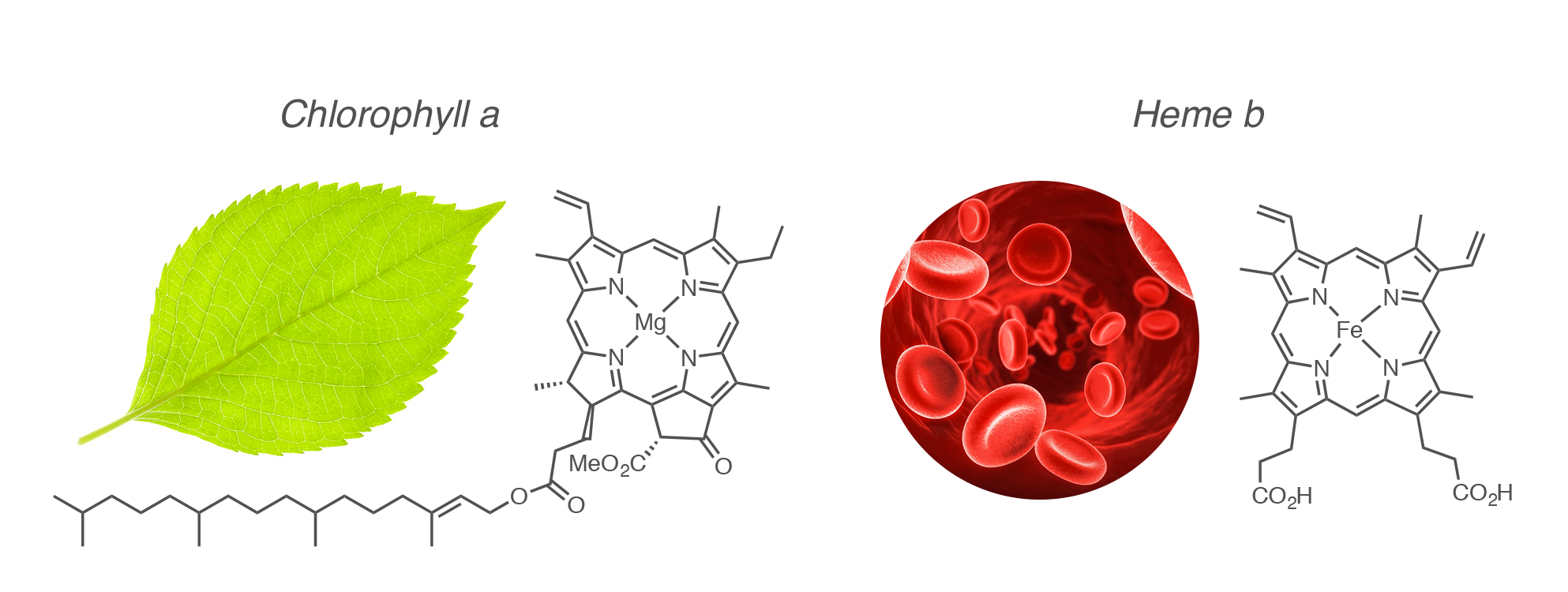
In nature, porphyrins and related tetrapyrrolic derivatives are involved in major life processes such as photosynthesis and the respiratory chain. Excellent chelating agents, they are involved in these processes through their metal complexes. The chlorophyll, a partially reduced magnesium(II) porphyrin, is the main actor of the conversion of the sunlight energy into chemical energy in photosynthetic organisms. The heme, an iron(II) porphyrin, is the active site of hemoglobin responsible for the transportation of dioxygen in erythrocytes (red blood cells); Heme is also involved in myoglobin responsible for the storage of dioxygen in muscle cells as well as in a, b and c cytochroms, which serve as redox intermediates in electron transfer processes.

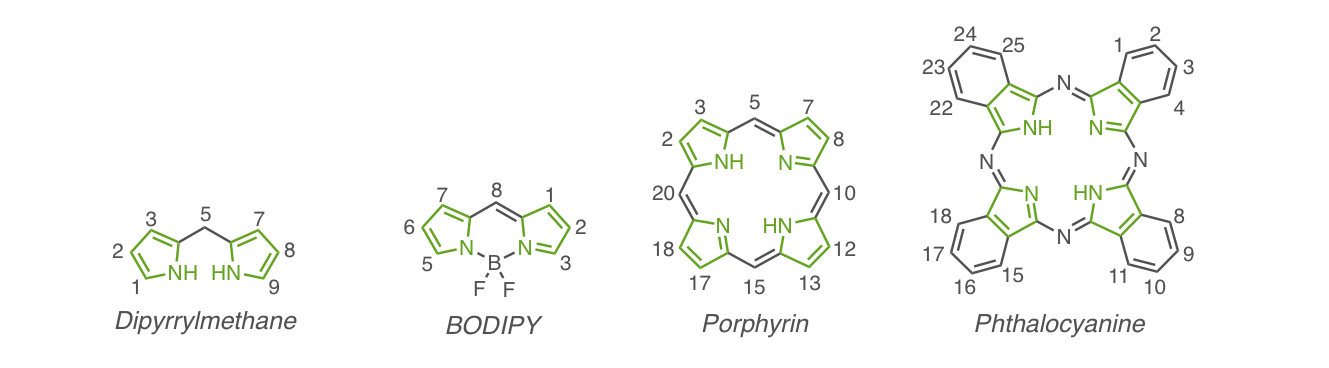
Classification
Through their aromatic structure, the porphyrins are very stable molecules compared with other organic dyes or metal complexes. Their aromatic system consists of 22 π–electrons of which 18 are involved in the aromaticity providing them particular spectroscopic properties as a strong absorption in the visible and the near–UV for instance. Their absorption spectrum consists in one intense band (≈ 105 M–1.cm–1) between 390 nm and 430 nm called Soret band, and several bands, ten to one hundred times less intense, located between 480 nm and 700 nm, called Q bands. Depending on the structure of the tetrapyrrolic compound, emission spectrum consists in 2 bands located between 650 nm and 750 nm. Compared with porphyrins, their analogs such as phthalocyanines exhibit very high extinction coefficients in a wavelength range that extends to around 700 nm. The porphyrins and related tetrapyrrole compounds represent the most important class of photosensitizers in natural photosynthesis. Hence, they are very attractive building–blocks for the construction of light-harvesting systems.
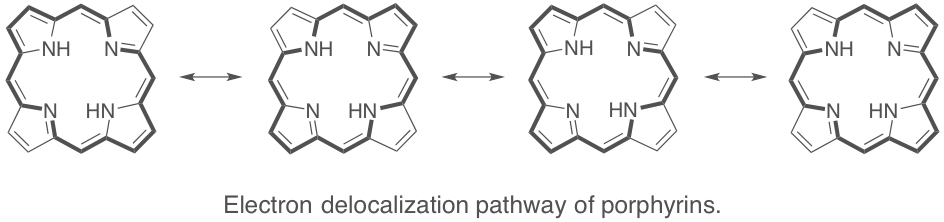
Porphyrins and related macrocycles are electroactive compounds involved in multiple redox processes, whose diversity depends on the presence of metal, the type of macrocycle and the type of axially coordinated ligand. The wealth of porphyrin electrochemical properties makes them efficient systems for the processes involving electron transfer reactions.
Deprotonated, porphyrins and phthalocyanines are some excellent tetradentate ligands. They can be metalated by almost all the metals from the periodic table by reaction with the corresponding metal salts. In biological systems, the properties of metalloporphyrins often depend on the nature of the coordinated metal. Under its dianionic form, the four electron pairs of the nitrogen atoms are oriented toward the center of the macrocycle. Thus, the metal is often located at the center of the porphyrin cavity. The minimum coordination number of the metal is equal to four but it can increase to eight thanks to the coordination in axial positions. Axial positions play a central role in the reversible axial ligation of adducts.
The scientists were soon interested in the porphyrin structure for the elaboration of molecular architectures in order to mimic the properties of natural systems. Their photosensitive characteristics coupled with their chelating capabilities make porphyrins, phthalocyanines and related compounds efficient systems for reversible axial ligation of adducts, electron transfer, light-harvesting and catalytic transformations that are crucial for the development of sustainable and green responses to modern societal challenges. Nowadays, porphyrins, phthalocyanines and related compounds are used in several applications in the fields of health, energy, smart materials and chemical processes.
Deprotonated, porphyrins and phthalocyanines are some excellent tetradentate ligands. They can be metalated by almost all the metals from the periodic table by reaction with the corresponding metal salts. In biological systems, the properties of metalloporphyrins often depend on the nature of the coordinated metal. Under its dianionic form, the four electron pairs of the nitrogen atoms are oriented toward the center of the macrocycle. Thus, the metal is often located at the center of the porphyrin cavity. The minimum coordination number of the metal is equal to four but it can increase to eight thanks to the coordination in axial positions. Axial positions play a central role in the reversible axial ligation of adducts.
The scientists were soon interested in the porphyrin structure for the elaboration of molecular architectures in order to mimic the properties of natural systems. Their photosensitive characteristics coupled with their chelating capabilities make porphyrins, phthalocyanines and related compounds efficient systems for reversible axial ligation of adducts, electron transfer, light-harvesting and catalytic transformations that are crucial for the development of sustainable and green responses to modern societal challenges. Nowadays, porphyrins, phthalocyanines and related compounds are used in several applications in the fields of health, energy, smart materials and chemical processes.


